Randomized Controlled Trial of the MySmartSkin to Promote Sun Protection
This report is taken directly from my PowerPoint Presentation
Introduction
There are approximately 90,000 cases of malignant melanoma that require diagnosis, making it the fifth most diagnosed cancer, with incidents typically increasing over time.
Purpose
The goal of this research is to conduct a randomized controlled trial to a new intervention (MySmartSkin) to determine skincare behavior amongst cancer patients
Project Overview
We conducted this project into 2 phases:
- Phase 1 focuses on the development the MySmartSkin intervention
- Phase 2 focuses on a randomized controlled trial of the MySmartSkin
Phase 1
The MySmartSkin intervention underwent development and refinement, incorporating a web-based online survey where participants provide information about their cancer status.
Phase 2
The goal is to assess the outcomes of MySmartSkin compared to the standard care for skin cancer patients. Patients were selected based on specific characteristics and assigned to randomized groups for the study.
Process Summary
Patients are chosen based on their stage of skin-cancer and are randomized to either the treatment or control groups
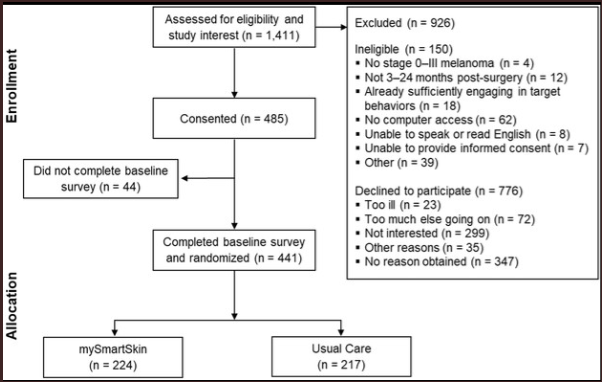
Treatment group gets the MySmartSkin whereas control group gets the usual-care treatment on their skin cancer. Results were measured through surveys every 8 weeks, 24 weeks, and 48 weeks later.
The statistical methods employed in this trial include Randomized Controlled trial, T-tests, and Chi-Squared tests aimed at examining differences in demographic and clinical characteristics among individuals.
Results
- Total (N = 287 )
- mySmartSkin intervention (n = 139 )
- Usual care (n = 148 )
| Body Area | Examined (%) | Examined (n) | Examined (%) | Examined (n) | Examined (%) | Examined (n) |
|---|---|---|---|---|---|---|
| Scalp | 37.9 | 108 | 39.6 | 55 | 36.3 | 53 |
| Face | 98.3 | 282 | 99.3 | 138 | 97.3 | 144 |
| Neck | 89.8 | 256 | 90.7 | 126 | 89.0 | 130 |
| Shoulders | 88.2 | 253 | 87.8 | 122 | 88.5 | 131 |
| Front of arms | 97.9 | 280 | 98.6 | 137 | 97.3 | 143 |
| Back of arms | 79.8 | 229 | 78.4 | 109 | 81.1 | 120 |
| Chest | 93.7 | 268 | 94.2 | 130 | 93.2 | 138 |
| Stomach | 87.7 | 250 | 87.7 | 121 | 87.8 | 129 |
| Upper back | 54.9 | 157 | 55.1 | 76 | 54.7 | 81 |
| Lower back | 48.8 | 139 | 50.0 | 69 | 47.6 | 70 |
| Front of legs | 94.0 | 266 | 93.4 | 128 | 94.5 | 138 |
| Back of legs | 72.6 | 204 | 71.3 | 97 | 73.8 | 107 |
| Bottom of feet | 41.6 | 119 | 40.6 | 56 | 42.6 | 63 |
| Buttocks | 40.4 | 116 | 39.6 | 55 | 41.2 | 61 |
| Genitals | 44.4 | 127 | 42.0 | 58 | 46.6 | 69 |
Of the 441 patients that consented to the trial
- N1=224 were placed into the treatment group, assigned MySmartSkin
- N2=217 patients were placed in the control group which is basically standard treatment
From the results of the intervention (MySmartSkin), we see that certain body parts showed a reduction in tumor size; however, the difference is not too significant
Conclusion
While there is some reduction in skin tumor size for the treatment group, the observed difference is not too significant; further research is needed on MySmartSkin to comprehensively test and evaluate its effectiveness.